Who We Are
We are MedTec freaks for 15 years already. The focus and the experience are the basic for excellence
Got the ISO 13485 (Medical devices) certification 6 months after we started and were recertified yearly.
Finalized more than 100 projects, more than 20 Class III (AIMD – Active Implants)
Team has an exquisite balance between experienced developers and young wolves
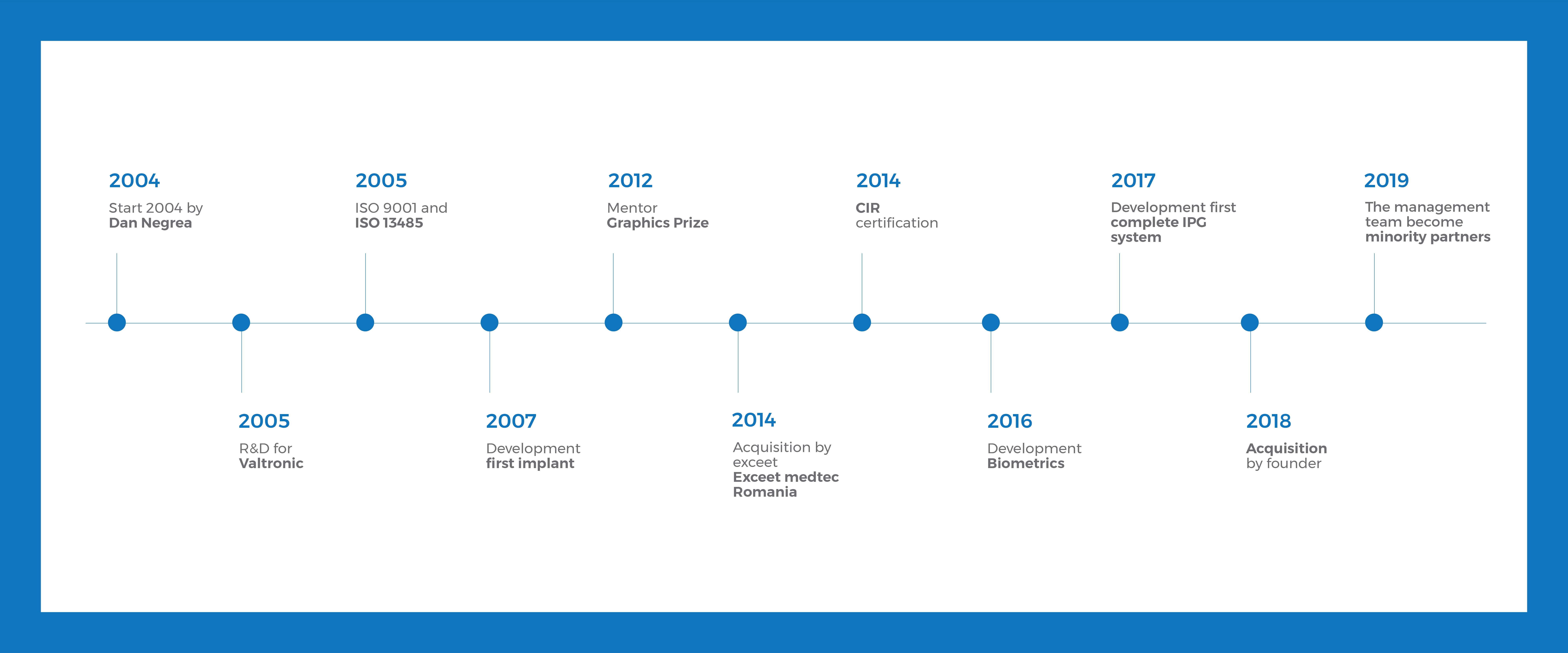
Started in 2004 as dedicated development center, the team based in Bucharest, is mature, experienced, reliable and flexible.
We grew since the exceet spin-off by 50% and got international: Romania, Germany, France, Switzerland
Our History
Mission
When starting a new Medtec project, your Core Competence is irreplaceable. We take care of the rest.
From concepts to requirements, from traceability of the documents to linked risk analysis, covering the whole development process and the assembly of complete products
Why work with us?
Experience
We learned a lot in 15 years of activity, more than 100 projects, more than 20 Class III Implants. This solid background know-how, at your disposition, will help increase the speed of the project and aim directly for the best solution
Dedicated to Medtec
Our focus on MedTec development offers an increased level of competency in service of your projects and speeds up the development without sacrificing the risk-based approach. The offered documenting infrastructure, the integrated tools for device life-cycle and risk management, all grant the legal manufacturer a smooth certification process
IP Protection
All the product related Foreground IP created within a project belongs to our customer. We supply all results in readable and in native format, thus removing possible exit barriers. All activity is covered by strict NDA.
Wide Spectrum
The development of a Medical Device requires a concerted approach, combining multiple disciplines, from ASIC to App, from Industrial Designer to Qualified Product. Within our team we cover Mechanic, Optic, Electronic development, PCB Design, Software, Firmware, RF, Acoustic, Industrialization and many more
Interdisciplinary
Innovations are interdisciplinary. For years already we foster the cross-cluster thinking and acting. By combining the competencies within a global vision, we focus on the relation between parts of the system or the underlying technology
Proximity
- Cultural and Geographic closeness
- EU centered Company, combining the presence in the Western part of the continent with the Near-Shore supply
- Communication in the major European languages
- Products are "Made in the EU" therefore no customs barriers
Testimonials
News
Asssembly
Evolution Medtec is expanding its services of medical device development by offering the assembly of small and medium series devices.
Our strategic planning for the future relies on the completion of the high-level development offer and manufacturing of prototypes by the integration of assembly capacities. This assembly capacities focus on small and medium volumes of devices, having the series production status.
Building on the 10+ years long R&D ISO 13485 certification, all assembly operations will be done in the manufacturing certified environment. This includes the setup of the logistic chain, documentation and traceability, control of the subcontractors as well as the permanent care for quality and pricing.
The assembly environment fulfills the ISO 8 standards.
We are in the process of investing in dedicated and universal equipment, like laser marking systems, CNC, dispensing equipment, electronic rework stations.
New team for regulatory affairs
Following the development path of our customers, we decided to strengthen our quality management and quality insurance team, by adding three new members to our team.
The core activity where they will be involved concerns the rapid and competent handling of operational quality management and quality insurance, including handling of engineering change orders for our customers providing documentation for the CE compliance, performing risk and ergonomic analysis.
Our Jobs
We are in permanent search for talents therefore we strongly encourage you to send your letter of intent and CV.
We promise you a fast response and are glad to engage in a discussion.
Our development in the future relies on the acquisition of new projects – and there are multiple projects in the pipeline – so, opportunities for cooperation arise rapidly.




